The cell membrane cannot be considered a homogenous system from the stand point of lipids or proteins since both types of molecules constitute assemblies of varying temporal and spatial stability. The hierarchical association of membrane proteins represent such a structure, but lipid microdomains also belong to them. Lipid rafts are such supramolecular organization of lipids, which are thermodynamically unstable, small (10-100 nm) structures. Proteins, lipids, the cytoskeleton and membrane turnover all contribute to their generation. They are similar, but not identical, in many respects to the liquid-ordered (Lo) domains in model membranes. The lipid environment of the cell membrane obviously influences the biophysical and cell biological properties of transmembrane proteins through their transmembrane domain. An important property of the cell membrane is the dipole potential, which is positive potential of magnitude 200-500 mV in the hydrophobic interior of the cell membrane generated by the dipoles of lipids and membrane-associated water molecules. Since the transmembrane domains of membrane proteins also have a dipole, the two dipoles interact with each other.
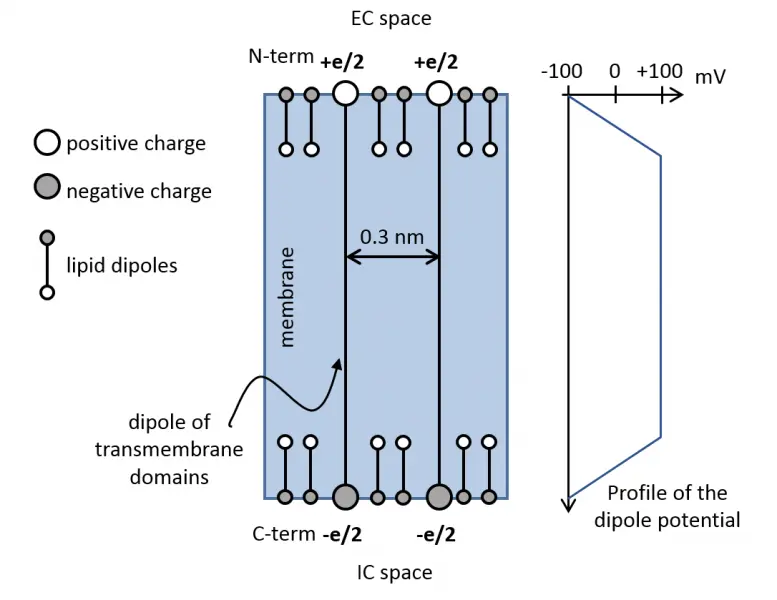
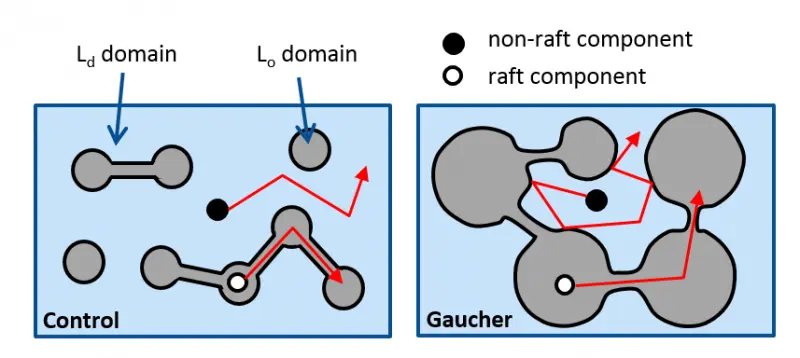

We have established that
- the dipole potential significantly enhances the ligand-induced clustering and signaling of ErbB1 and ErbB2
- the dipole potential is biologically significantly larger in lipid rafts than in other parts of the membrane
- increasing the sphingolipid content of the cell membrane enhances the size of liquid-ordered (Lo) domains and “traps” lipid and protein constituents residing in the liquid-disordered (Ld) domain inhibiting their mobility and function. Such an increase in the sphingolipid content of the cell membrane is present in Gaucher’s disease.

In our current research efforts we are seeking an answer for how systematic alterations in the lipid composition of the cell membrane change the behavior (clustering, mobility, signaling) of membrane proteins.
Last update:
2023. 06. 21. 11:48