Voltage-gated ion channels are proteins in cellular plasma membranes that respond to membrane potential changes to regulate numerous cellular functions such as excitability, transport processes, proliferation and apoptosis. Malfunctioning channels can cause life-threatening diseases, and many drug molecules target ion channels to treat a variety of diseases.
Detailed knowledge of the molecular structure of the channel protein is essential to understand the gating mechanism and how it is affected by external factors. This comprehensive view of the structure and function is necessary to shed light on the pathomechanism of channelopathies and for the rational design of drugs to treat such disorders.
The general architecture of voltage-gated K+ (Kv) channels is a tetramer of identical subunits that each contain six membrane-spanning helices (S1-S6, Fig.1). Helices S1-S4 make up the voltage-sensing domain (VSD) where S4 has a prominent role in the sensor function by containing multiple residues with positive side chains. Depolarization of the membrane causes outward movement of S4, which is transmitted to the pore domain (PD) formed by S5-S6 and the pore loop connecting them and opens the conduction pathway.
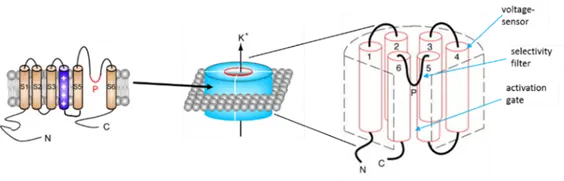
Figure: Voltage-gated K+ channels are made up of four subunits each of which contains six alpha helices (S1-S6). S1-S4 form the voltage sensor domain, while the S5-S6 helices from each subunit together form the conducting pore.