Ion channel function can be regulated by the membrane lipid environment. Beyond the obvious physical changes that the alteration of the lipid composition causes, such as in fluidity and mechanical stress, lipids can also interact with ion channels in less general ways, for example by rearranging the microdomain structure, modifying local electric potential profiles or even specifically binding to them. In certain diseases (Smith-Lemli-Opitz syndrome, Gaucher’s disease) the lipid composition of the cell membranes may change, which in turn affects the embedded ion channels. As an essential lipid building block of the cellular plasma membrane in the human organism, cholesterol is also involved in these phenomena. In addition to its structural role, it regulates the fluidity and raft structure of the membrane and influences the course of numerous membrane-linked signaling pathways and the function of transmembrane proteins, including ion channels. From our previous studies we know that cholesterol can modify the gating of Kv channels by shifting their voltage-dependence and slowing their opening and decreasing the current, so the channel pore is affected. Our goal is to identify the target of cholesterol-induced changes: does it affect the voltage-sensor, which is then transmitted to the pore, or is it the pore that is directly affected, or maybe the coupling between the two domains? We are using different lipids and Kv channels to determine the specificity of the phenomena.
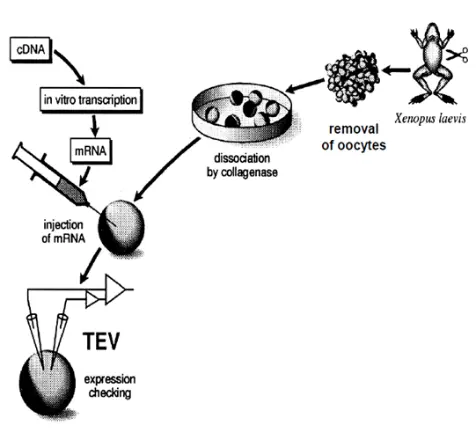 | 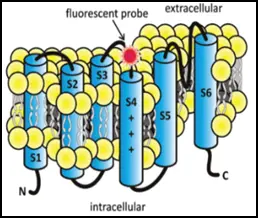 | 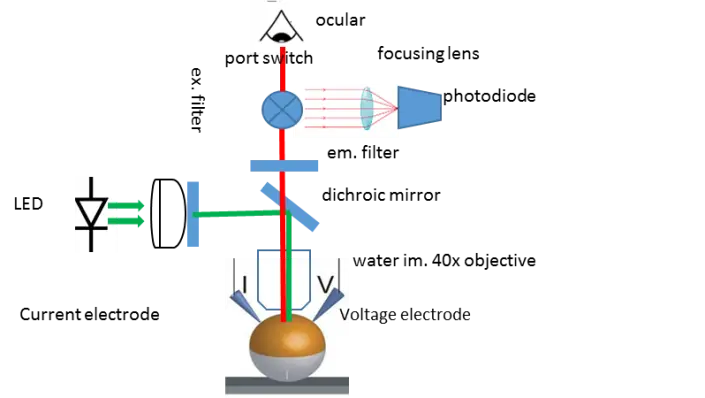 |
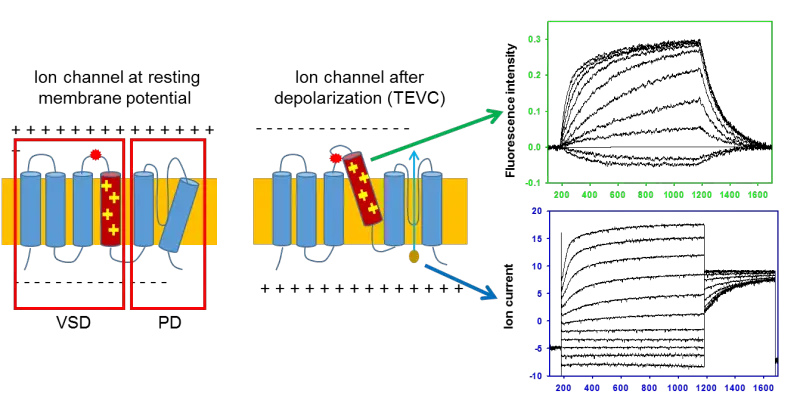
Figure: In Voltage-clamp Fluorometry frog oocytes are injected with the RNA of the channel to be studied. A cysteine residue is inserted in the sequence to label it with a fluorescent dye. The membrane potential of the cell is controlled by two electrodes and the current through the channels is measured. The rearrangement of the voltage-sensor is followed by the change in the fluorescence of the dye. This allows independent observation of the pore (current) and the voltage-sensor (fluorescence).