The basic research carried out by the researchers of Biophysics and Cell Biology Department can help in drug design and may contribute to a more effective chemotherapy treatment of tumors. The results of this multi-year study were recently published in eLife, a prestigious international scientific journal.
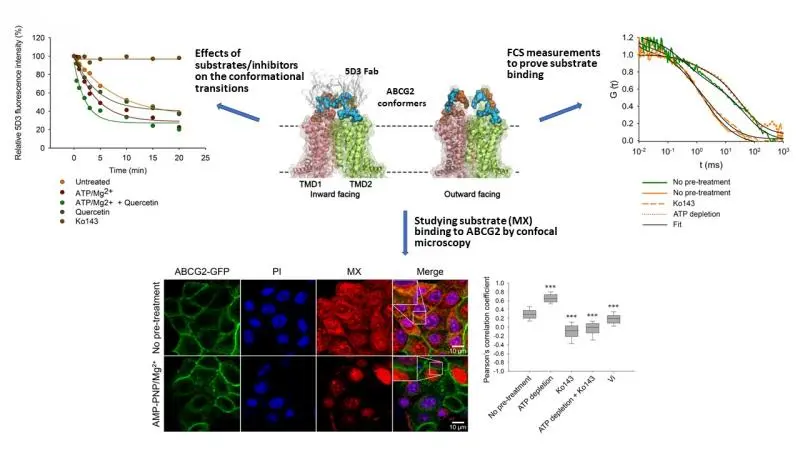
ABCG2 is an active transporter-type human ABC protein that affects the pharmacokinetics of numerous chemotherapeutic drugs used for the treatment of various diseases and an important player of the chemotherapy resistance of tumors. Based on recent structural studies ABCG2 fluctuates between two major conformational states upon its duty cycle: in the inward-facing (IF) state the substrate binding site is accessible from the intracellular space, while in the outward-facing the substrate binding site is open from the extracellular space. However, it is not known how is the IF to OF transition coupled to binding and hydrolysis of ATP. The conformation changes of ABCG2 were followed by measuring the binding of a conformation selective antibody, while substrate binding to the transporter was studied by fluorescence correlation spectroscopy (FCS) and confocal microscopy-based fluorescence co-localization measurements. The experiments were carried out in live cells or semi-permeabilized cells, where ABCG2 molecules are present in their quasi-natural environment, in the undisturbed context of the plasma membrane.
The experiments demonstrate that the switch from the IF to the OF conformation of ABCG2 is induced by nucleotide binding. IF-OF transition is facilitated by substrates, and hindered by the ABCG2 inhibitor Ko143. Direct measurements of substrate binding to ABCG2 indicate that the high-to-low affinity switch of the drug binding site making possible the uphill transport of substrates coincides with the transition from the IF to the OF conformation. Low substrate binding persists in the post-hydrolysis state, supporting that dissociation of the ATP hydrolysis products is required to reset the high substrate affinity IF conformation of ABCG2. The experimental data also give insight to the kinetics of catalytic transitions.
The results of the study were published in eLife, a prestigious international scientific journal.