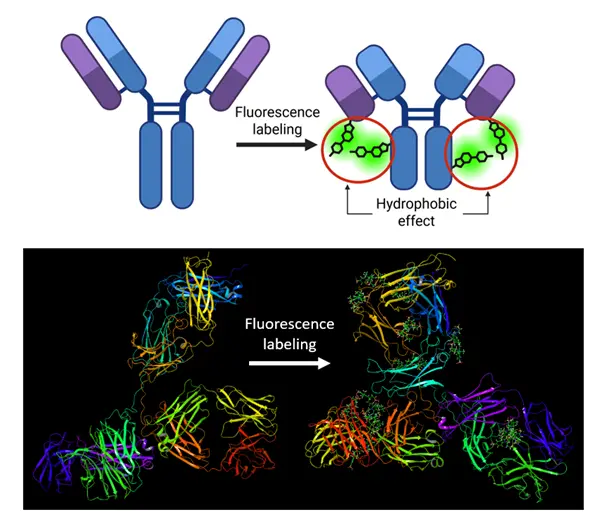
 Fluorescently labeled antibodies are used extremely widely in cell and molecular biology research. The method requires the covalent attachment of low molecular weight fluorescent dyes to the antibody. Although this chemical modification is easy to perform, it is known to reduce antibody affinity. While antigen recognition is the best-known function of antibodies, several other molecules, e.g., protein A, protein G, Fc gamma receptors, also bind to antibodies. Measurements by Tímea Hajdu and colleagues show that these functions are impaired almost as severely as antigen recognition. From this, the authors concluded that the phenomenon is driven by a global transformation of antibody structure and dynamics associated with fluorescence labeling. They verified this assumption using multiple biophysical techniques. Time resolved fluorescence anisotropy revealed that antibodies with a high degree of labeling exhibit faster dynamics in the tens of nanoseconds range. Antigen binding by fluorescently labeled antibodies is enthalpically less favorable and entropically less punished than that of unlabeled antibodies. Förster resonance energy transfer measurements demonstrated that highly labeled antibodies “collapse”, i.e., the distance between their Fc and Fab regions decreases. This conclusion was confirmed by molecular dynamics simulations, which reproduced such global structural changes in fluorescent antibodies (see the accompanying figure). Analysis of the simulations indicates that hydrophobic interactions between the fluorescent dyes attached to the antibody underlie the process. Glycerol partially inhibited the adverse effect of fluorescence labeling on antibody affinity. This combined experimental and simulation study lays the groundwork for developing labeling methods that impair antibody biological functions to a lesser extent. The authors reported their results in the International Journal of Biological Macromolecules (https://doi.org/10.1016/j.ijbiomac.2025.146209).
Fluorescently labeled antibodies are used extremely widely in cell and molecular biology research. The method requires the covalent attachment of low molecular weight fluorescent dyes to the antibody. Although this chemical modification is easy to perform, it is known to reduce antibody affinity. While antigen recognition is the best-known function of antibodies, several other molecules, e.g., protein A, protein G, Fc gamma receptors, also bind to antibodies. Measurements by Tímea Hajdu and colleagues show that these functions are impaired almost as severely as antigen recognition. From this, the authors concluded that the phenomenon is driven by a global transformation of antibody structure and dynamics associated with fluorescence labeling. They verified this assumption using multiple biophysical techniques. Time resolved fluorescence anisotropy revealed that antibodies with a high degree of labeling exhibit faster dynamics in the tens of nanoseconds range. Antigen binding by fluorescently labeled antibodies is enthalpically less favorable and entropically less punished than that of unlabeled antibodies. Förster resonance energy transfer measurements demonstrated that highly labeled antibodies “collapse”, i.e., the distance between their Fc and Fab regions decreases. This conclusion was confirmed by molecular dynamics simulations, which reproduced such global structural changes in fluorescent antibodies (see the accompanying figure). Analysis of the simulations indicates that hydrophobic interactions between the fluorescent dyes attached to the antibody underlie the process. Glycerol partially inhibited the adverse effect of fluorescence labeling on antibody affinity. This combined experimental and simulation study lays the groundwork for developing labeling methods that impair antibody biological functions to a lesser extent. The authors reported their results in the International Journal of Biological Macromolecules (https://doi.org/10.1016/j.ijbiomac.2025.146209).
Fluorescence labeling causes global changes in antibody structure and dynamics through the hydrophobic effect
Fluorescence labeling causes global changes in antibody structure and dynamics through the hydrophobic effect
Last update:
2025. 08. 22. 14:10