Kenesei Á, Volkó J, Szalóki N, Mocsár G, Jambrovics K, Balajthy Z, Bodnár A, Tóth K, Waldmann TA, Vámosi G. J Immunol. 2021 Oct 15:ji2100277. doi: 10.4049/jimmunol.2100277
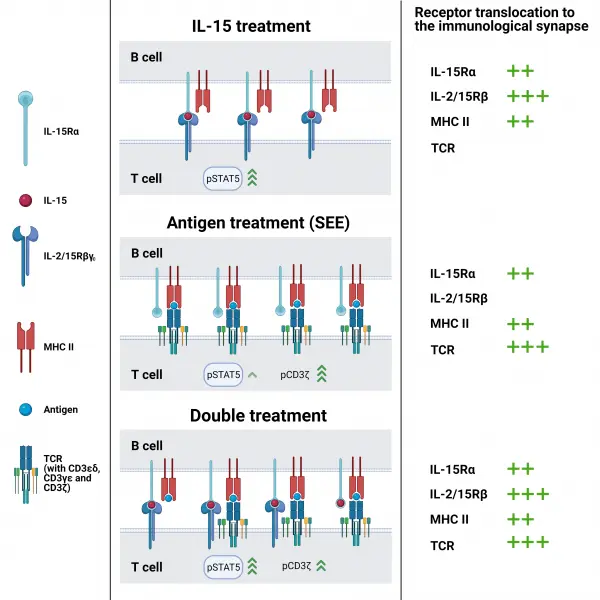
Interleukin-15 (IL-15) plays a key role in long-term survival of memory T-cells, thus being a central player in immunological memory. Unlike most cytokines, IL-15 uses trans-presentation (TP) for ligand delivery. During IL-15 TP IL-15 receptor α chain, located on the presenting cell, presents IL-15 to the IL-2/15Rβ and γc chains located on the responding T/NK cell. Antigen presentation (AP), the first step of T cell activation, takes place between an APC and a T cell in a similar manner. It is an intriguing question whether the two processes are linked with each other or are independent. Therefore, we investigated the effect of AP on IL-15 TP and vice versa. We detected enrichment of IL-15Rα and IL-2/15Rβ at the synapse and positive FRET efficiency if presenting B cells were pre-treated with IL-15, giving the first direct biophysical evidence for IL-15 TP. IL-15Rα and MHC II interacted and translocated jointly to the immunological synapse when either ligand (antigen or IL-15) was present, whereas IL-2/15Rβ and CD3 moved independently of each other. IL-15 TP initiated STAT5 phosphorylation in responding cells (T cell), which was not further enhanced by AP. Conversely, IL-15 treatment slightly attenuated antigen-induced phosphorylation of CD3ζ chain. Our results show that IL-15 TP can be considered an autonomous, antigen-independent process.