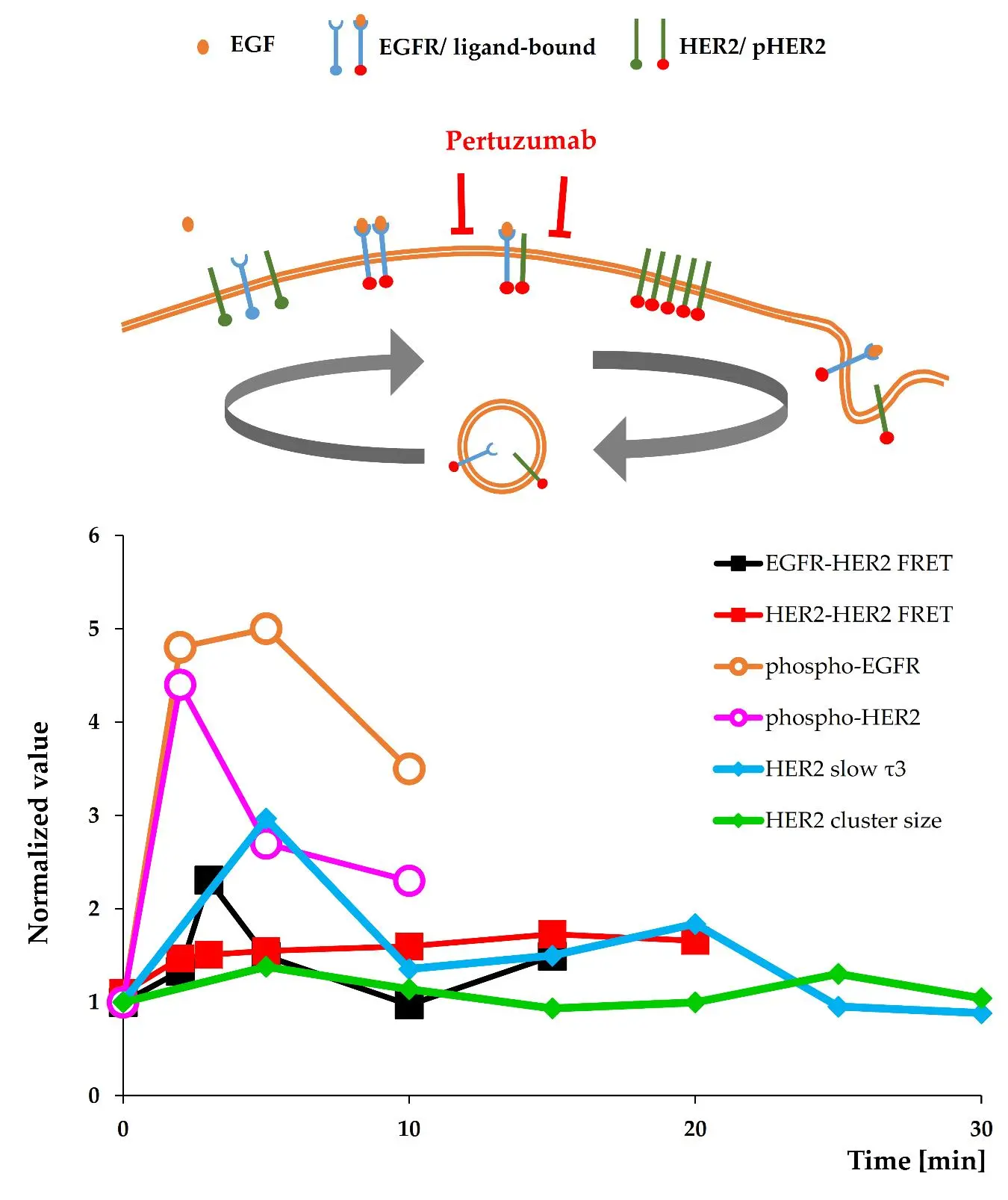
 Ligand- induced formation of signalling platforms composed of homo- and heterodimers of the ErbB/HER-family is considered essential for the origin and progression of many cancers. Upon EGF binding, the Epidermal Growth Factor Receptor (EGFR) acts as a signal receiver and, together with its ligandless heterodimerization partner, Human Epidermal growth factor Receptor 2 (HER2), produces strong mitogenic input for many cells. Their interaction plays a key role in various malignancies, especially when HER2 molecules are overexpressed. Ligand-driven transactivation is a key step leading to changes in the cell surface pattern of EGFR and HER2. Our clinically relevant model system is the SK-BR-3 breast-tumor cell line, which overexpresses HER2, moderately expresses EGFR, and shows dependency on EGF-driven HER2 signalling. EGFR-HER2 interaction in the cell membrane upon EGF binding, followed by an increase in HER2 homoassociation, was studied using various biophysical approaches on different time scales. Changes in molecular proximity were characterized by fluorescence lifetime imaging microscopy (FLIM) techniques assessing Förster resonance energy transfer (FRET), confirming the ligand-enhanced interaction and association of these receptors. EGF binding and transactivation were reflected also in the phosphorylation of both receptors. In parallel, superresolution (Airyscan) microscopy and fluorescence correlation and cross-correlation spectroscopy (FCS/FCCS) revealed cyclic increases in the aggregation and stable co- diffusion of membrane- localized HER2, possibly caused by internalization and recycling, eventually leading to a new equilibrium in the cell membrane. Overall, the complementary array of state-of-the-art imaging cytometry approaches demonstrates a spatiotemporal pattern of spontaneous and induced aggregation states of HER2 modulated by the presence of ligand-bound EGFR. Of particular significance, the time course of EGFR-HER2 heteroassociation and the dynamics of HER2 homoclustering suggests that in the case of EGFR-triggered HER2 transactivation, there may be time-windows when the therapeutic antibody pertuzumab can more efficiently bind to its target and exert its effect.
Ligand- induced formation of signalling platforms composed of homo- and heterodimers of the ErbB/HER-family is considered essential for the origin and progression of many cancers. Upon EGF binding, the Epidermal Growth Factor Receptor (EGFR) acts as a signal receiver and, together with its ligandless heterodimerization partner, Human Epidermal growth factor Receptor 2 (HER2), produces strong mitogenic input for many cells. Their interaction plays a key role in various malignancies, especially when HER2 molecules are overexpressed. Ligand-driven transactivation is a key step leading to changes in the cell surface pattern of EGFR and HER2. Our clinically relevant model system is the SK-BR-3 breast-tumor cell line, which overexpresses HER2, moderately expresses EGFR, and shows dependency on EGF-driven HER2 signalling. EGFR-HER2 interaction in the cell membrane upon EGF binding, followed by an increase in HER2 homoassociation, was studied using various biophysical approaches on different time scales. Changes in molecular proximity were characterized by fluorescence lifetime imaging microscopy (FLIM) techniques assessing Förster resonance energy transfer (FRET), confirming the ligand-enhanced interaction and association of these receptors. EGF binding and transactivation were reflected also in the phosphorylation of both receptors. In parallel, superresolution (Airyscan) microscopy and fluorescence correlation and cross-correlation spectroscopy (FCS/FCCS) revealed cyclic increases in the aggregation and stable co- diffusion of membrane- localized HER2, possibly caused by internalization and recycling, eventually leading to a new equilibrium in the cell membrane. Overall, the complementary array of state-of-the-art imaging cytometry approaches demonstrates a spatiotemporal pattern of spontaneous and induced aggregation states of HER2 modulated by the presence of ligand-bound EGFR. Of particular significance, the time course of EGFR-HER2 heteroassociation and the dynamics of HER2 homoclustering suggests that in the case of EGFR-triggered HER2 transactivation, there may be time-windows when the therapeutic antibody pertuzumab can more efficiently bind to its target and exert its effect.
DOI: https://doi.org/10.1002/cyto.a.24922