 Nucleosomal structure is, in general, repressive for transcription, replication, and repair, thus one strategy of eukaryotic cells to regulate these activities involves de-repression by destabilizing or mobilizing particular nucleosomes. As such, the stability of nucleosomes is of utmost regulatory importance, and it is assumed to be modulated by posttranslational modifications (PTMs) on histones, by the reader proteins binding to them as well as by histone variant composition. In this context, destabilizing effects are expected in the case of activating functions, while nucleosome stabilization would suit repressive roles. In a puzzling manner, there are observations suggesting that the presence of the H2A.Z variant in nucleosomes can increase, or decrease nucleosome stability.
Nucleosomal structure is, in general, repressive for transcription, replication, and repair, thus one strategy of eukaryotic cells to regulate these activities involves de-repression by destabilizing or mobilizing particular nucleosomes. As such, the stability of nucleosomes is of utmost regulatory importance, and it is assumed to be modulated by posttranslational modifications (PTMs) on histones, by the reader proteins binding to them as well as by histone variant composition. In this context, destabilizing effects are expected in the case of activating functions, while nucleosome stabilization would suit repressive roles. In a puzzling manner, there are observations suggesting that the presence of the H2A.Z variant in nucleosomes can increase, or decrease nucleosome stability.
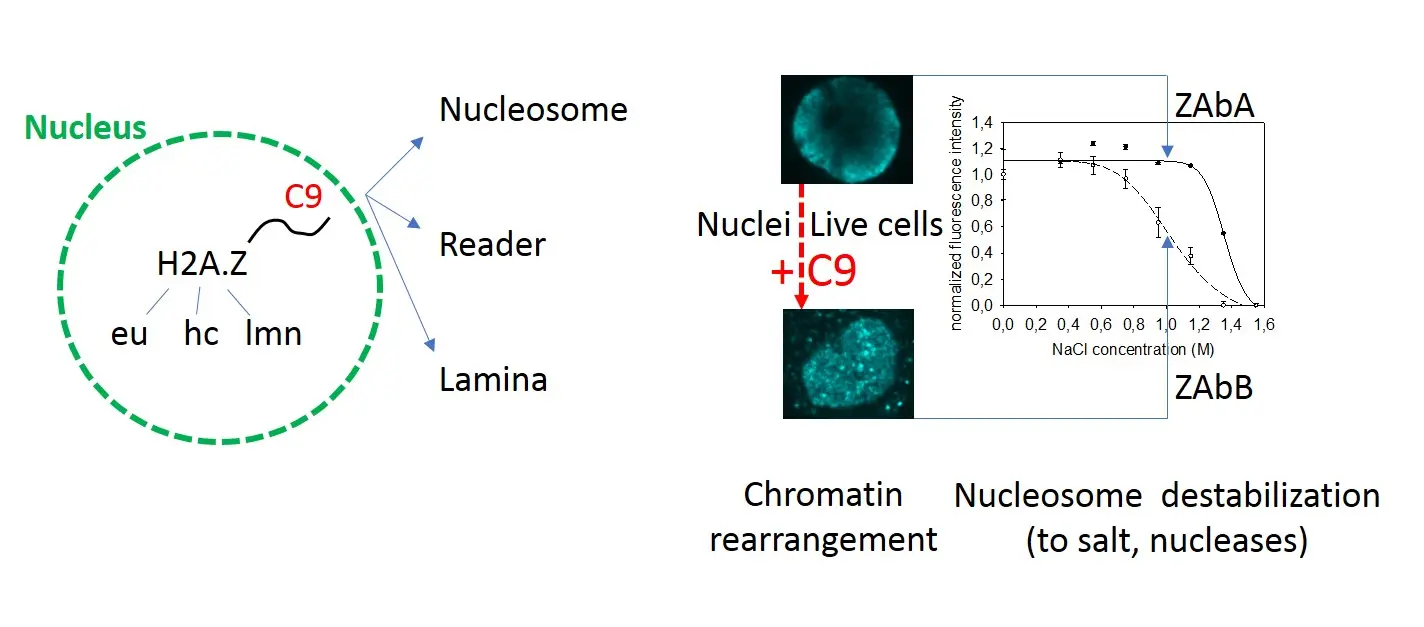
In this paper, nucleosome stability was measured in a robust in situ assay to assess its molecular determinants in H2A.Z-containing nucleosomes. The method (Imre L, et al. Nucleosome stability measured in situ by automated quantitative imaging. Scientific reports 7, 12734 (2017)) involves exposure of the nucleosomes in agarose-embedded nuclei to a concentration series of salt and measuring the histones of interest remaining in the nuclei by immunofluorescence and quantitative imaging, using antibodies specific for particular histones, histone variants or their posttranslational modifications (PTMs). This approach has led us to recognize a remarkable heterogeneity of H2A.Z-containing nucleosomes, independently from their isotype composition: three types can be observed, euchromatic (H2A.Zeu), heterochromatic (H2A.Zhc) and lamina-associated (H2A.Zlmn).
The primary observation was that a large fraction of H2A.Z detected by four different antibodies (H2A.Zhc) was released from the nucleosomes by salt together with H3, in contrast with the much less stably bound H2A.Zeu, H2A or H2B. Furthermore, this unusual behavior relied on the presence of the unstructured C-terminal chain of the histone variant, and was unaffected by H2A.Z1 or H2A.Z2 isoform specificity, histone PTMs or the H2A.Z reader protein PWWP2A, as determined using cell lines expressing only particular forms of the variant and hydroxyapatite chromatography of reconstituted nucleosome arrays. In the absence of the tail, or upon addition of an excess of the tail peptide to the nuclei of control cells, the canonical H2A-like stability features prevailed or were readily restored as most of the H2A.Z-containing nucleosomes were observed as scattered foci in the nuclei rather than exhibiting the peripheral topography typical for H2A.Zhc. The organization of H3K9me3-marked constitutive heterochromatin as well as of the DNA itself within the native chromatin were also distinguishable in the nuclei of cells expressing wild type or tailless H2A.Z, and were rearranged upon addition of the tail peptide (C9) to isolated nuclei of cells harboring the wild type. Chromatin reorganization was accompanied by an increased overall sensitization to salt as well as to nucleases, i.e. destabilization. Increased MNase sensitivity could be demonstrated equally within H3K4me3-marked euchromatic and H3K9me3-marked heterochromatic domains. Thus, interactions involving a short H2A.Z peptide chain simultaneously determine the stability and accessibility features of chromatin involving the nucleosomes containing this histone variant, encompassing large chromatin regions in the nucleus.
The study was published in a prestigious international scientific journal, Nature Communications.
László Imre, Péter Nánási Jr, Ibtissem Benhamza, Kata Nóra Enyedi, Gábor Mocsár, Rosevalentine Bosire, Éva Hegedüs, Erfaneh Firouzi Niaki, Ágota Csóti, Zsuzsanna Darula, Éva Csősz, Szilárd Póliska, Beáta Scholtz, Gábor Mező, Zsolt Bacsó, H T Marc Timmers, Masayuki Kusakabe, Margit Balázs, György Vámosi, Juan Ausio, Peter Cheung, Katalin Tóth, David Tremethick, Masahiko Harata, Gábor Szabó
DOI: 10.1038/s41467-024-53514-9