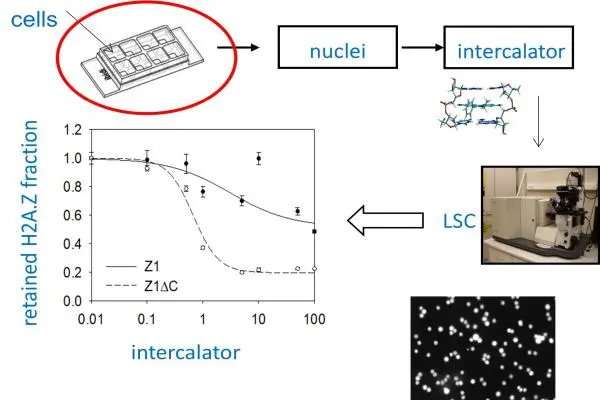
 H2A.Z-containing nucleosomes can assume either a stable or an unstable configuration depending on the presence or absence of the C-terminal nonapeptide of the variant histone (Z1 or Z1∆C, respectively). These observations were made using an in situ microscopic assay developed by the authors involving exposure of native chromatin to intercalator compounds that bind to and unwind the DNA, changing its superhelicity similarly to what happens during transcription and DNA replication in the live cell. These experiments have led to the hypothesis that binding of the terminal peptide to the DNA may depend on its superhelicity, a model corroborated by the comparison of the diffusion properties of the fluorescent dye-labeled synthetic peptide in the presence of superhelical and relaxed plasmid DNA molecules as measured by fluorescence correlation spectroscopy. In view of the localization of a subclass of these nucleosomes at the transcription start sites, the observation raises the possibility that the stability of the strategic +1 nucleosome is regulated by the topological state of the promoter. The study has been published recently in Nucleus (https://pubmed.ncbi.nlm.nih.gov/40926315/).
H2A.Z-containing nucleosomes can assume either a stable or an unstable configuration depending on the presence or absence of the C-terminal nonapeptide of the variant histone (Z1 or Z1∆C, respectively). These observations were made using an in situ microscopic assay developed by the authors involving exposure of native chromatin to intercalator compounds that bind to and unwind the DNA, changing its superhelicity similarly to what happens during transcription and DNA replication in the live cell. These experiments have led to the hypothesis that binding of the terminal peptide to the DNA may depend on its superhelicity, a model corroborated by the comparison of the diffusion properties of the fluorescent dye-labeled synthetic peptide in the presence of superhelical and relaxed plasmid DNA molecules as measured by fluorescence correlation spectroscopy. In view of the localization of a subclass of these nucleosomes at the transcription start sites, the observation raises the possibility that the stability of the strategic +1 nucleosome is regulated by the topological state of the promoter. The study has been published recently in Nucleus (https://pubmed.ncbi.nlm.nih.gov/40926315/).
H2A.Z-nucleosomes are stabilized by the superhelicity-dependent DNA binding of the C-terminal tail of the histone variant
H2A.Z-containing nucleosomes can assume either a stable or an unstable configuration depending on the presence or absence of the C-terminal nonapeptide of the variant histone (Z1 or Z1∆C, respectively).
Last update:
2025. 10. 10. 12:32