Bálint Rehó, Gábor Mocsár, Lina Fadel, Péter Brázda, László Nagy, György Vámosi and their colleagues from Germany, Lukas Lau, Gabriele Müller and Katalin Tóth developed together a microscopic method, and examined the dimerization and DNA-binding of nuclear receptors in live cells. The method (SPIM-FRET-FCCS) can be used to simultaneously map the proximity (using Förster resonance energy transfer, FRET), the joint motion and the mobility (using fluorescence cross-correlation spectroscopy, FCCS) of fluorescently tagged molecules in a selected plane of the cell, thus it is suitable for studying protein-protein and protein-DNA interactions. Retinoic acid receptor (RAR) binds to DNA and regulates the transcription of its target genes in a ligand-dependent manner, and serves as a therapeutic target in several diseases. It was demonstrated by the new method that agonist ligand enhances RAR dimerization with the retinoid X receptor and also increases the dimer’s DNA binding. The paper was published in the journal Analytical Chemistry doi: 10.1074/jbc.RA119.011614 (IF: 6.78).
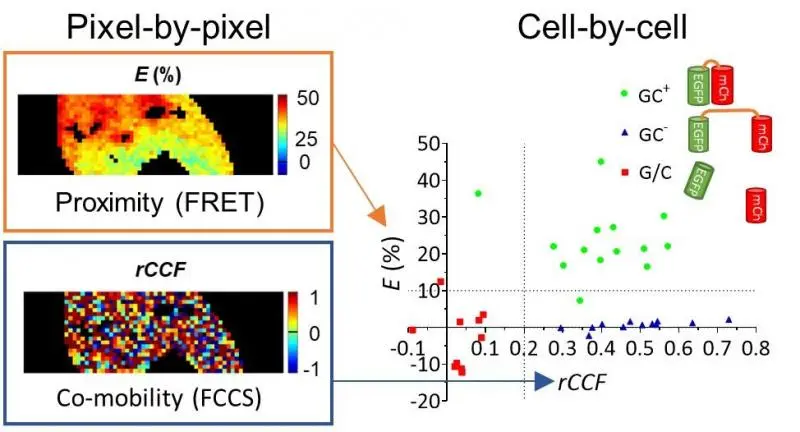
Picture: Demonstrating the distinction of coexpressed protein pairs based on their proximity and co-mobility. Left: 2D maps of proximity (E: FRET efficiency) and co-mobility (rCCF: apparent co-diffusing fraction) measured from a GFP-mCherry fusion protein (GC+) connected by a short linker. Right: Cellular median values distinguishing high FRET, high comobility (GC+), low FRET high comobility (GC-: dyes connected by a long linker) and low FRET, low co-mobility (G/C: separately expressed dyes) populations.